You are here : ILVENResearch TeamsSORGFluorinated compounds and photoinduced processes
- Partager cette page :
- PDF version
Fluorinated compounds and photoinduced processes
Coordinator of the axis
Permanent members
Elsa Anselmi (Associate Professor, orcid)
Guillaume Dagousset (CNRS Researcher, orcid)
Patrick Diter (Associate Professor)
Bruce Pégot (Associate Professor, orcid)
Christine Thomassigny (Associate Professor)
PhD students/Postdocs
Camille Banoun (PhD student)
Marina Briand (PhD student)
Arnaud De Zordo-Banliat (PhD student)
Yang Li (PhD student)
Augustin Nouaille (Post-Doc)
Julien Paut (PhD student)
Francesco Terzani (Post-Doc)
Main themes
One of the major objectives in this area concerns the search for mild, late-stage selective methods for the introduction of fluorinated groups into organic molecules. New perfluoroalkylation methods and/or new methods of introduction of fluorinated groups have been described, as well as their application for the synthesis of chosen targets in the fields of synthetic methodology, medicinal chemistry or in materials chemistry. We also try to develop new photoinduced transformations, in particular by means of photoredox catalysis, for the synthesis of original motifs and structures.
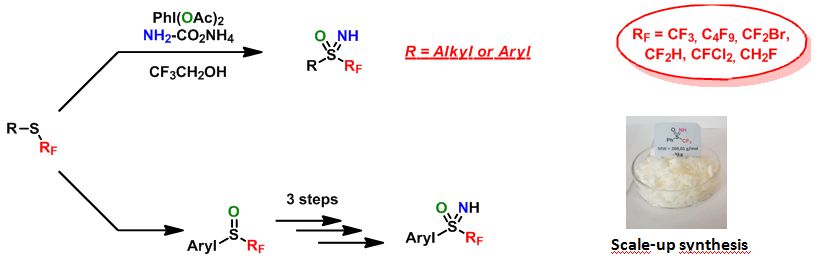
Fluorine and sulfur: thioethers, sulfoxides, sulfoniums, sulfilimines, sulfilimino iminiums and sulfoximines
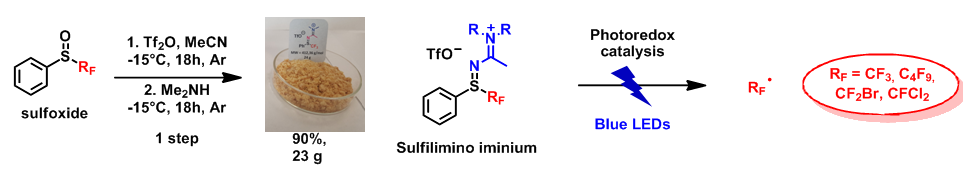
We have developed a simple, efficient and divergent method which leads to a choice of targets: to sulfilimines, sulfilimino iminiums or to sulfoximines, novel compounds with potential applications as reagents or as innovative moieties in biological chemistry. The principal results concern post-functionalisation studies of these new molecules and their properties. Numerous new sulfoximines have been synthesised. We have particularly looked at functionalisation of the nitrogen atom of these sulfoximines, as well as of the aromatic ring substituent of the sulfur. Concerning the properties of these compounds, this family has been identified as a very efficient source of perfluorinated radicals by photoredox catalytic activation. The sulfilimino iminiums, which are the simplest compounds to prepare, are by far the best candidates for this radical chemistry.
Perfluoroalkylation by photoredox catalysis
A successful collaboration with Dr. Masson (ICSN, Gif-sur-Yvette) focused on multi-component reactions has been established within the Labex Charm3at. This methodology leads by oxo-, carbo- and amino-trifluoromethylation of ene-carbamates to the synthesis of many novel fluorinated molecules. Photocatalysed amino-trifluoromethylation has been extended to styrenes and olefins, by adapting the experimental conditions. These conditions have also allowed the use of aromatic and heteroaromatic nucleophiles with benzylic cations, as well as halogenated nucleophiles (chlorine, bromine and iodine).
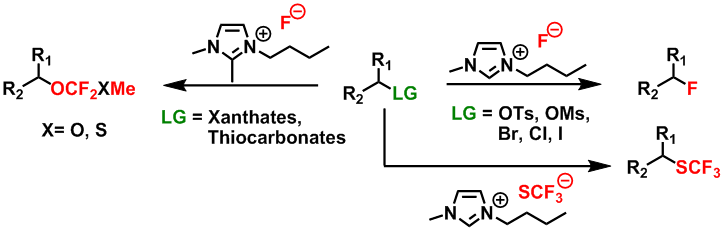
New reaction media for eco-compatible chemistry (ionic liquids)
Our approach consists of using ionic liquids simultaneously as solvents as well as nucleophilic fluorination reagents. The compounds [bmim][F], [bdmim][F] et [bmim][SCF3] have been used respectively for nucleophilic fluorinations, fluorodesulfuration reactions and for nucleophilic trifluoromethylthiolations.
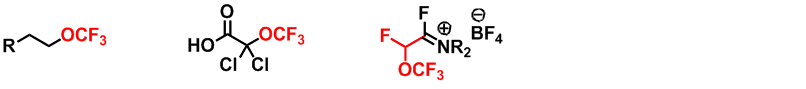
New emergent fluorinated substituents (OCF3)
Two main areas have been developed within this topic. The first concerns the preparation of highly functionalised new molecules that may act as synthons for the production of more complex compounds. The second concerns a new FAR type reagent (in collaboration with Dr. F Leroux, LIMA Strasbourg). This reagent allows the electrophilic introduction of a carbon substituted with both a fluorine atom and an OCF3 group.
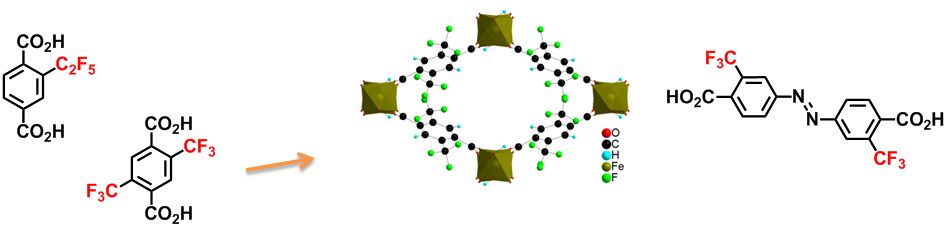
Fluorinated Ligands for porous solids
In collaboration with the previous Porous Solids group, we prepared organic ligands to modify the organic scaffolds of MOFS with the aim of modifying the properties of these compounds. While the fluorinated groups had little effect on the adsorption of hydrogen, their steric hindrance produced notable effects on pore size. This greatly improved the adsorption of alkanes in the gaseous phase. Another promising and noteworthy result was the ability of MIL-53(Fe)-(CF3)2 to separate linear alkanes from branched alkanes.