You are here : ILVENResearch TeamsSORGMolecular Materials: from Design to Applications
- Partager cette page :
- PDF version
Molecular Materials: conception, design and applications
Coordinator of the axis
Permanent members
Emmanuel Allard (Associate Professor)
Olivier David (Associate Professor, orcid)
Hélène Fensterbank (Associate Professor)
Michel Frigoli (CNRS Researcher, orcid)
Karen Wright (CNRS Researcher)
PhD Students/Postdocs
Jason Bessonnet (PhD student)
Oumou Diallo (PhD student)
Matylde Kuchar (PhD student)
Abdelilah Takfaoui (ATER)
Main themes
This theme has set out to conceive novel molecular architectures, from their design and synthesis through to their applications. Over the last few years we have concentrated on fullerene chemistry, but also on aromatic molecular structures based on naphthalene, acene, perylene or their derivatives, exploiting the planarity of these pi-conjugated systems, or the spatial deformation of their 3D topologies. These systems have been studied at the molecular level, but also grafted onto surfaces or in the form of thin films.
Fullerene C60 chemistry
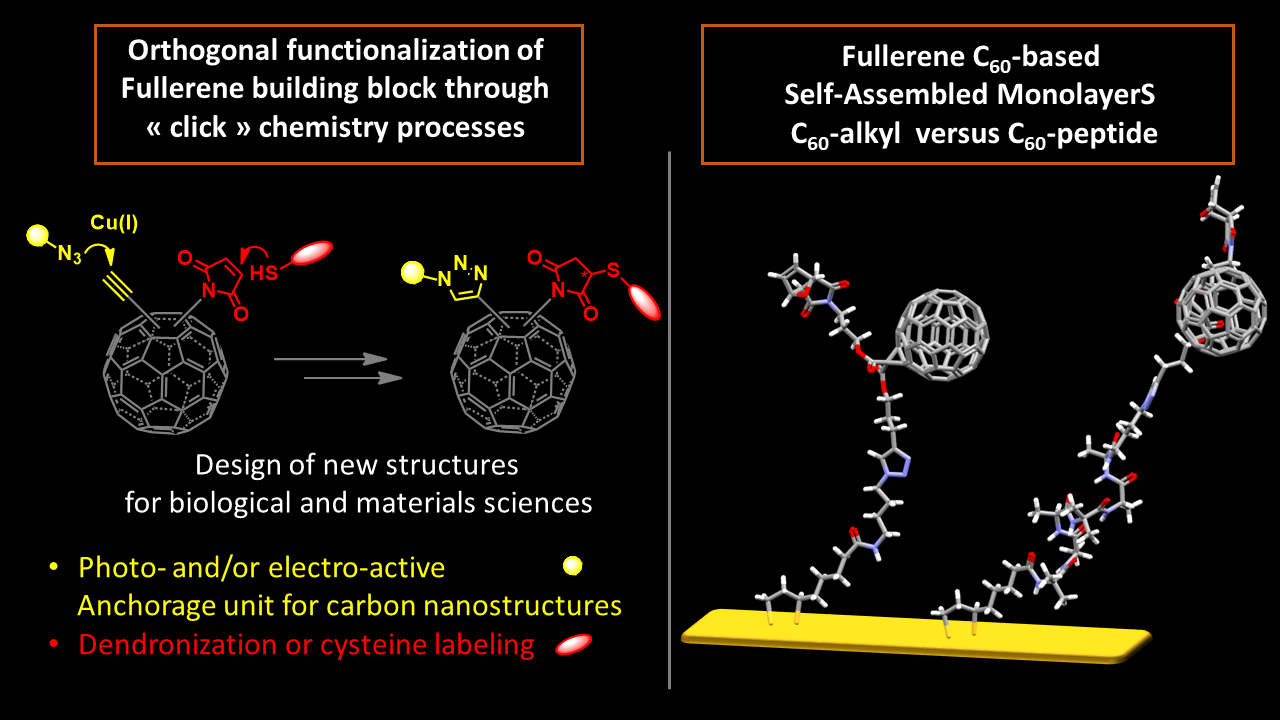
Organic semiconductors
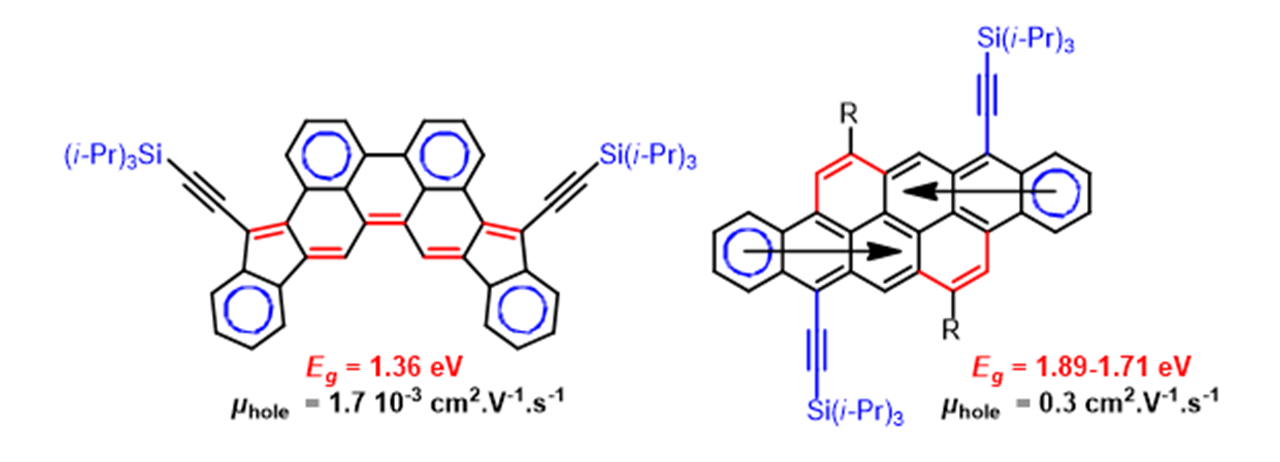
Polycyclic conjugated hydrocarbons are potentially interesting for the construction of optoelectronic devices. Generally, pi-conjugated systems which absorb at over 600nm are poorly stable to the combined action of oxygen and/or light. One of our objectives has been to develop molecules with a small HOMO-LUMO gap to be used as the active layer in field effect transistors. Recently, to achieve this aim, bis-acene type polycyclic hydrocarbon structures or quinoidal structures which incorporate (di)indeno-acene type anti-aromatic sub-units have been synthesized.
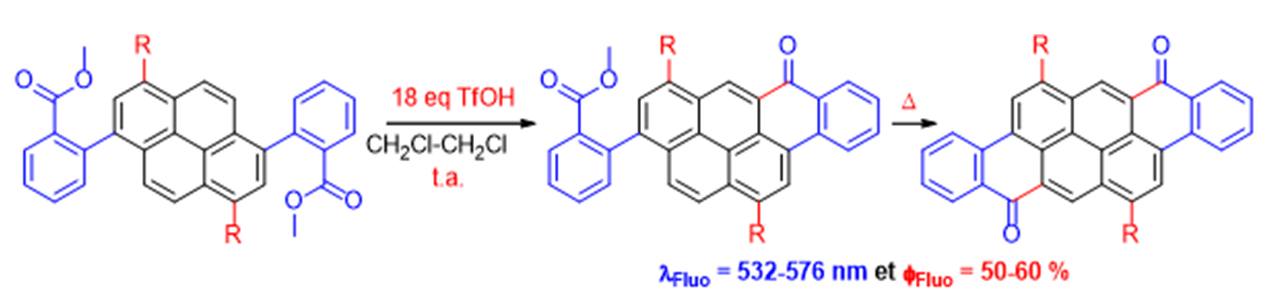
New fluorescent compounds have been synthesized from diversely functionalised pyrene dibenzoates by using intramolecular Friedel-Crafts reactions and triflic acid as activator. This acid allows control of the reactivity and regioselectivity to obtain the target molecules in excellent yields.
2D and 3D Aromatic architectures. Synthesis, modulations and properties
2D Topology. The planarity and/or the deformation from a plane of polyaromatic molecules have been exploited to generate structuration effects on surfaces, and control their optical, electronic and wetting properties.
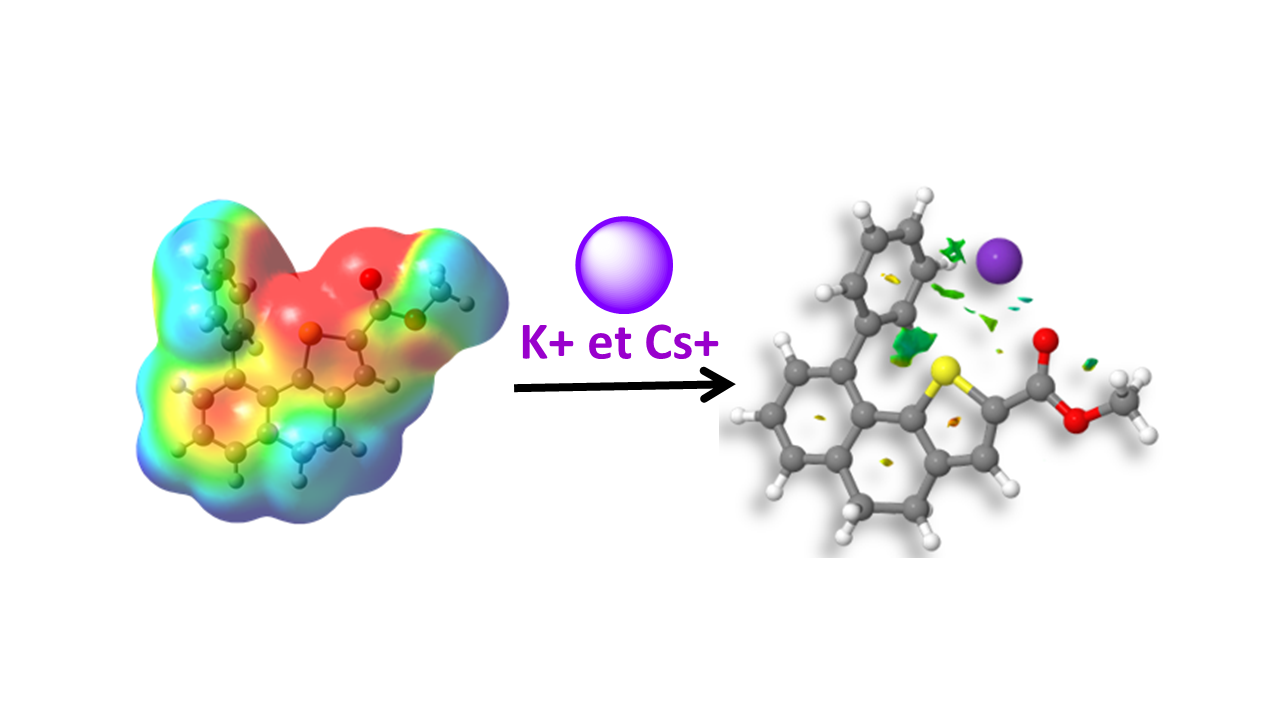
3D Topology. Topology control by using helicity parameters and selectively placing substituents or heteroatoms are key points for generating diversity in 3D molecular objects. A theoretical and experimental approach allowed us to show that selective cation (or anion) complexation resulted from the co-operative combination of different weak bonds (Pi-, S- and CO-cation).