You are here : ILVENResearch TeamsSORGFluorinated compounds and photoinduced processes
- Updated on October 18, 2024
- PDF version
Fluorinated compounds and photoinduced processes
Coordinator of the axis
Permanent members
Guillaume Dagousset (CNRS Researcher, orcid)
Patrick Diter (Associate Professor)
Bruce Pégot (Associate Professor, orcid)
PhD students/Postdocs
Main themes
One of the main concerns of this research area is the exploration of mild, late and selective methods for the incorporation of fluorinated groups into organic molecules. Innovative perfluoroalkylation reagents have recently been reported, as well as new approaches for the introduction of fluorinated entities, notably by photoredox catalysis. Major results focus on 3 main themes:
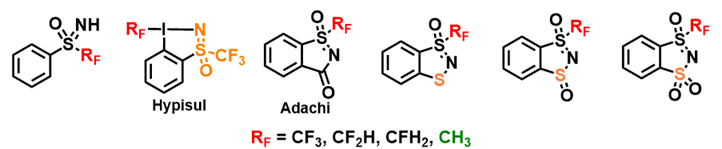
Sulfoximines and derivatives
We have developed a simple, efficient and innovative approach to the synthesis of sulfoximines, original and promising substances as reagents or innovative functional groups. The main results obtained recently concern the improvement of access routes to fluorinated sulfoximines, the development of new sulfoximines to improve their reactivities and the generalization of their applications in synthesis methodology. In particular, the ANR (PRCI) HYPISUL project, in partnership with Professor Antonio Togni (ETH Zürich), has enabled us to describe novel structures merging a hypervalent iodine with a fluorinated sulfoximine motif. This new reagent has enabled us to transform alcohols directly into the corresponding trifluoromethyl ethers, a reaction which is extremely delicate to achieve.
As far as modification of the aromatic ring is concerned, the use of the free NH sulfoximine directing group proved to be an effective strategy, enabling the introduction of numerous motifs in the ortho position. An NMR study revealed the species involved in this reaction. Sulfoximines bearing an alkyne function in the ortho position were able to undergo selective cyclization, giving either benzothiazines or benzothiazoles. The latter were then subjected to oxidation to obtain the Adachi reagent, which had previously only been mentioned in a Japanese patent dating from 2005. In collaboration with Dr. Samir Messaoudi (BIOCIS), it has been possible to connect to the aromatic ring of thioglycosides by two methods: palladium catalysis or dual catalysis (nickel-photoredox catalysis). These oxidation reaction compounds have enabled us to develop a novel family of analogues of the Adachi reagent. The introduction of a sulfur atom at different oxidation states modifies the properties of these skeletons, in comparison with Adachi's reagent, and makes them a good source of fluorinated radicals in photoredox catalysis.
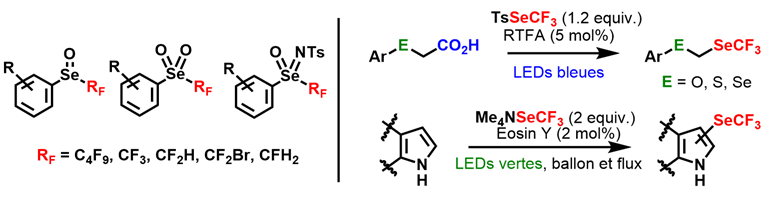
Selenium atom: SeCF3 and related oxidized species
By analogy with sulfoximines, fluorinated aromatic selenoximines had never before been described. A recent methodological study has given us access to an efficient method for the different oxidized states of perfluorinated selenoethers, namely selenoxides, selenones and selenoximines. This work was carried out as part of an ANR PRC project in collaboration with Dr. Thierry Billard's team (ICBMS Lyon). During this collaborative research, we developed the use of perfluoroalkylselenolation reagents. The reactivity of tosyl trifluoromethylselenyl (TsSeCF3) was explored. This derivative can participate in Morita-Baylis-Hillman-type reactions. Using mild, environmentally friendly conditions, we succeeded in synthesizing α,β-unsaturated ketones containing a SeCF3 motif. Initially electrophilic, the reactivity of TsSeCF3 could be reversed by generating in situ the CF3Se- anion (“umpolung” process), which could then react with bromoalkyls to form the corresponding selenoethers. Our expertise in photoredox catalysis also enabled us to develop a decarboxylating trifluoromethylselenolation reaction using tetraacetylated riboflavin as photocatalyst and TsSeCF3 as selenofluorinating agent. We were thus able to isolate new selenated fluorinated groups of the ECH2SeCF3 type and measure their lipophilicity (SeCH2SeCF3 having the highest known lipophilicity to date). In addition, photoredox catalysis, implemented without the use of metals but using eosin as a catalyst, proved highly effective in generating SeCF3 radicals for the first time from the nucleophilic trifluoromethylselenolation reagent, [Me4N][SeCF3]. This enabled the SeCF3 motif to be introduced on various heteroaromatic rings. The formation of radical species was demonstrated by spin trapping/EPR spectroscopy.
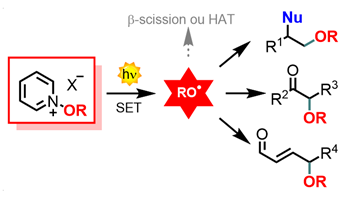
Photoredox catalysis
In addition to the new photocatalytic reactivities developed with our new reagents mentioned above (perfluoroalkylation, perfluoroalkylselenolation), a major axis of our research has focused on the generation of oxygenated radicals by photoredox catalysis. Thanks to the use of pyridinium salts as precursors for these radicals, we have been able to develop novel C-O bond radical formations, by trapping alkoxy radicals with suitable substrates such as alkenes or silylated enol ethers. The latter reactivity has also been generalized to the -OCF3 radical, in partnership with Professors A.Togni (ETH Zürich) and Luca Dell'Amico (University of Padova). Silylated dienol ethers, for their part, showed very interesting selectivity in favor of the γ-position, and this new type of γ-functionalization of enals could then be extended to other nitrogen or carbon radicals, as well as to perfluoroalkyl radicals thanks to our sulfoximines. It should also be noted that most of these photocatalytic reactions have been adapted to continuous flow chemistry, a technique which has also been used to develop Prins-type cyclization reactions. This theme is linked to the Catalyses and Heterocycles axis, in an attempt to develop an asymmetric version of these methods through organocatalytic activation.