You are here : ILVENResearch TeamsSORGCatalysis and heterocycles
- Partager cette page :
- PDF version
Catalysis and heterocycles
Coordinator of the axis
Permanent members
Bruno Drouillat (Associate Professor)
Anne Gaucher (Associate Professor, orcid)
Clément Ghiazza (CNRS Researcher, orcid)
Laurence Menguy-Le Roy (Associate Professor)
Damien Prim (Professor, orcid)
PhD Students/Postdocs
Oumayma Bejaoui (PhD student)
Florent Le Guern (IR CDD)
Tourya Khlifi (PhD student)
Sarra Ouni (PhD student)
Olfa Zayene (PhD student)
Main themes
Synthesis, characterisation and reactivity studies of innovative catalytic systems
A family of ligands containing the pyridylmethylamine group were shown to be very polyvalent, allowing the formation of C-C and C-O bonds by metal catalysis (Pd, Cu, Yb, Zn). The first examples of heterogeneous catalysis on biopolymer supports were described. These multi-purpose ligands are capable of creating a non-racemic environment around protons. They have been used in organocatalysis and in mixed catalytic cascade processes, proving the « relay ligand » concept.
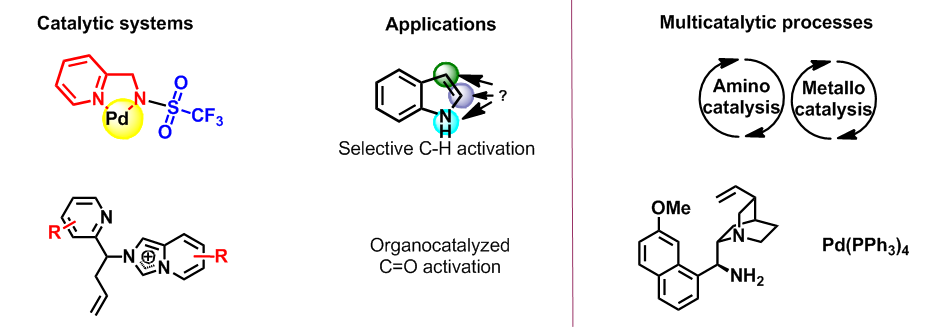
Development of original catalytic methods for the synthesis of complex molecular architectures
The design of efficient reaction sequences which can generate the formation of several chemical bonds in one step is a useful approach to synthesize rapidly complex and varied molecular structures. With this aim, new domino reactions relying on organo- or photocatalytic approaches for the synthesis of functionalised polycyclic architectures have been developed over the last few years.
Synthesis, reactivity and applications of N,O,S,P heterocycles
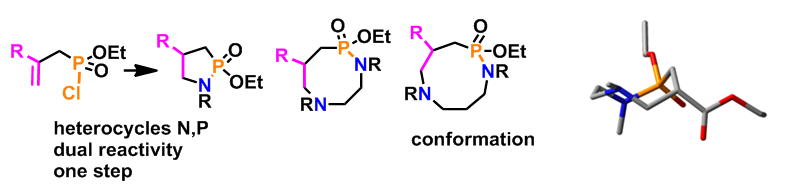
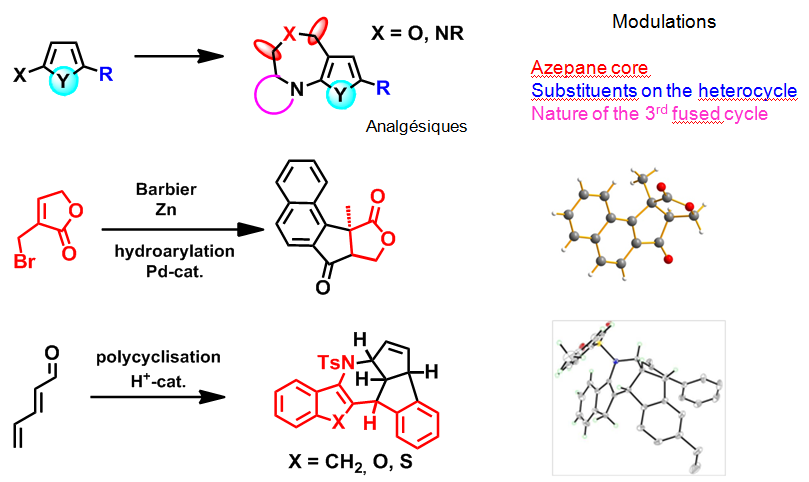
The synthesis and reactivity studies of saturated nitrogen heterocycles is a major part of our work. Several aspects of the varied reactivity of azetidines have been explored. New pathways to obtain tetrahydro-pyrans and -pyridines (currently undergoing biological testing), by a Prins cyclisation, or of phospho-nitrogen heterocycles of variable size (from 5 to 9 members) by the creation of an N-P bond followed by an aza-Michael type cyclisation have been developed. For some of these synthetic paths, the methodology has been completed by conformational space studies by NMR and by DFT calculations. In the aromatic series, the functionalisation of furans and thiophenes by a halogenation-Sonogashira sequence followed by intramolecular cyclisation, gave the targeted fused tricyclic compounds with a central oxazepine-type core.
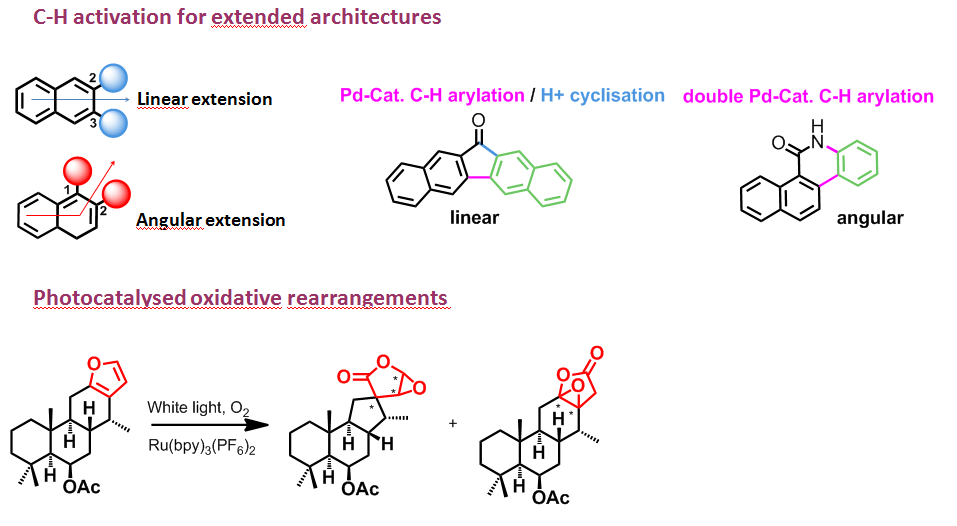
Construction of novel complex hetero(poly)cyclic compounds
We have shown that complex polyheterocyclic compounds may be rapidly obtained by a cascade of polycyclisations discovered in our laboratory. These three-dimensional heterocyclic structures are present in many interesting molecular architectures and have a wide field of biological applications.
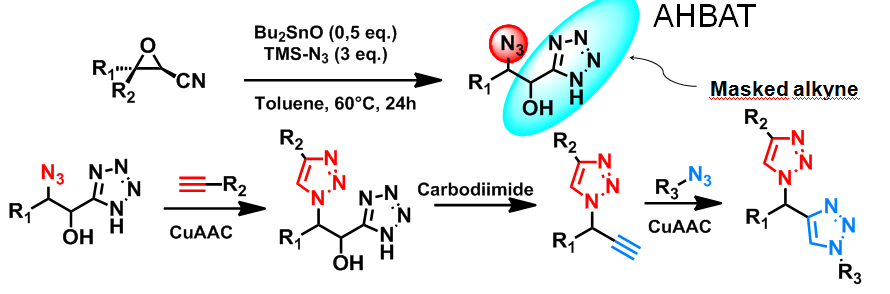
Triazoles from a single or double CuAAC reaction sequence
A novel reaction sequence which allows an easy access to triazole or bis-triazole compounds was recently developed. This strategy allows the synthesis of triazoles and bis-triazoles by two sequential CuAAC ligation steps. The two CuAAC reactions are independent and completely orthogonal. This sequential double ligation sequence, which may be iterative to form a polytriazole, was the subject of two patents.